Learn More About Our Programs
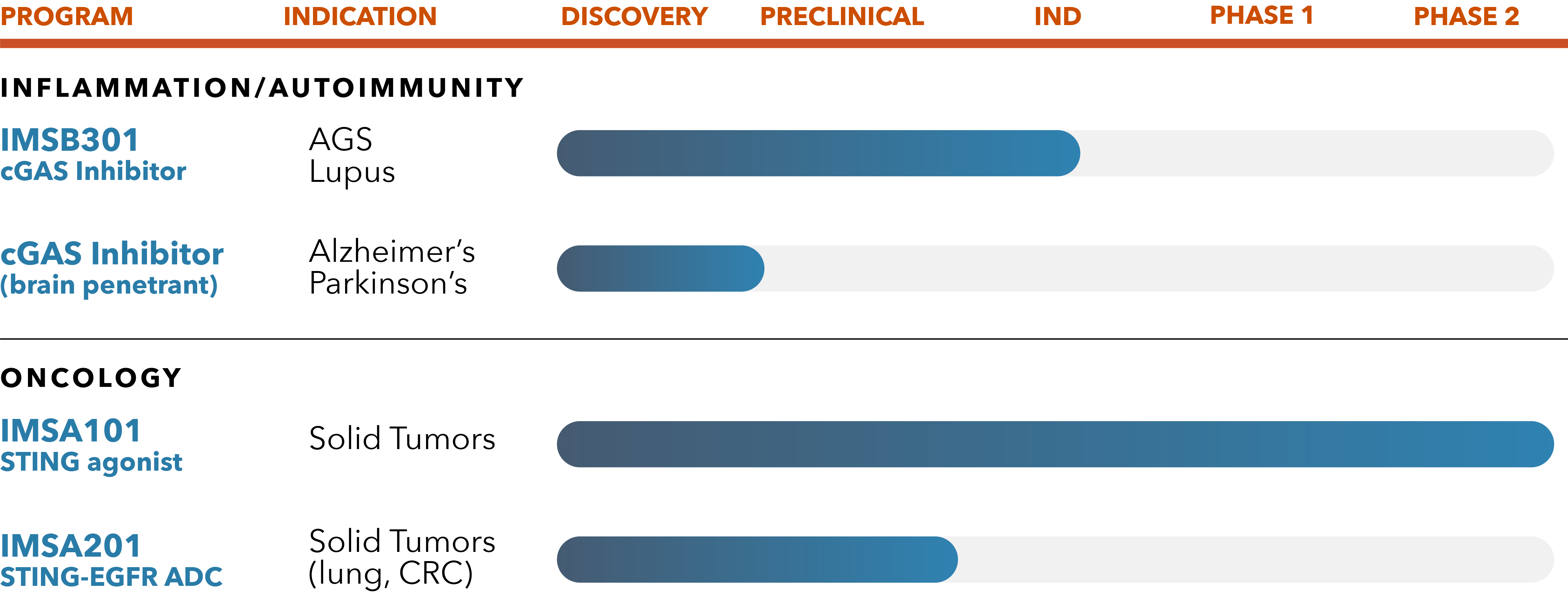
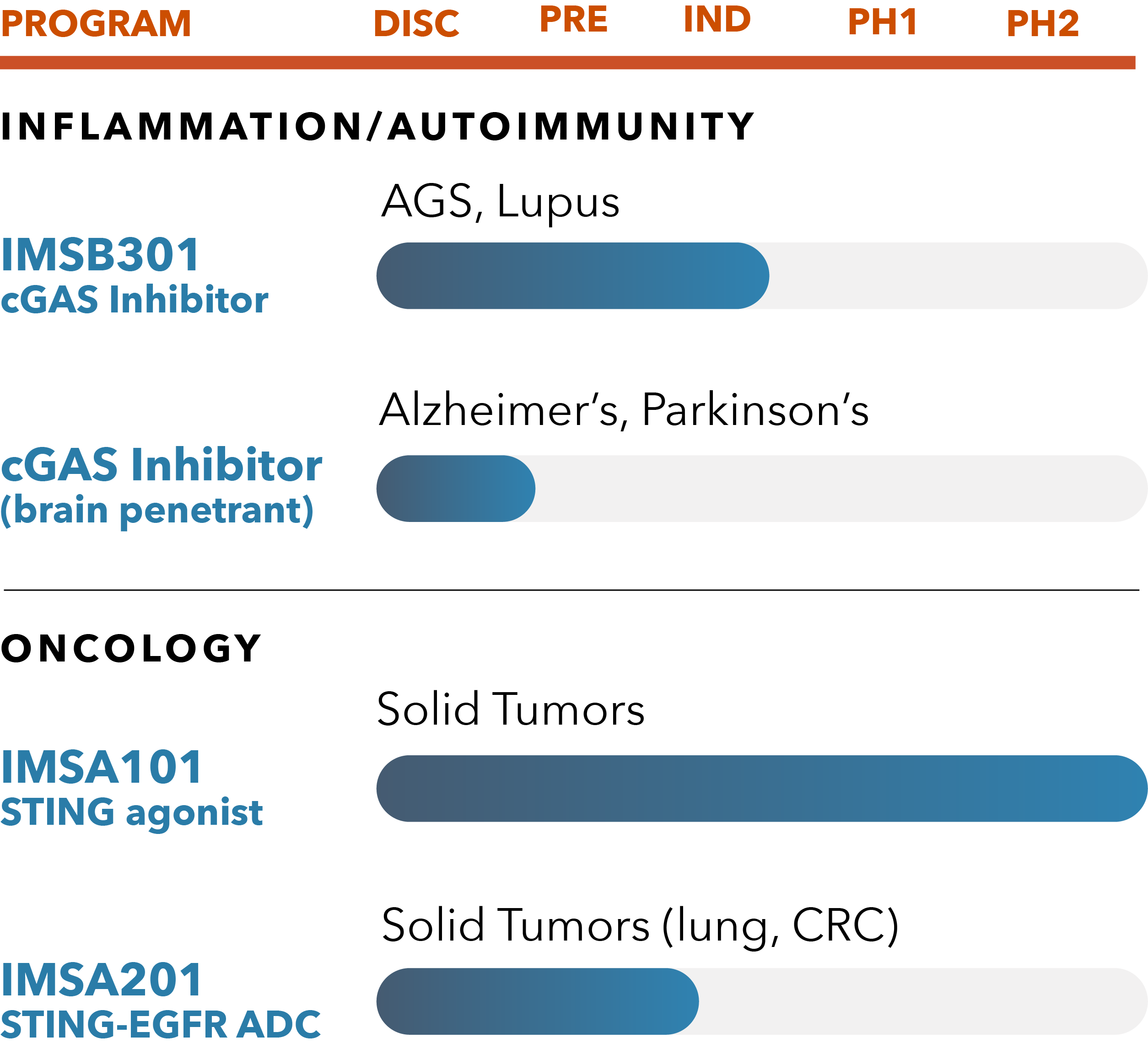
IMSB301 cGAS Inhibitors
Autoimmune disease arises when the body’s natural defense system cannot distinguish between its own cells and foreign cells, resulting in the body mistakenly attacking normal cells. Preventing aberrant activation of the cGAS-STING pathway is crucial for maintaining immune homeostasis. If the cGAS-STING pathway is chronically activated by lack of regulation, it causes activation of the immune system and inflammation, resulting in autoimmune diseases.
Several enzymes (RNase H2, TREX1, and DNase II) regulate cGAS activation by controlling the basal level of cytosolic DNA. Mice deficient in these functional enzymes have been shown to develop autoimmune disease with inflammation and lethality in a cGAS- and STING-dependent manner, underscoring a critical role for the cGAS-STING pathway in the pathogenesis of autoimmune diseases.
Based on in vitro and in vivo potency, DMPK and safety-toxicity studies, we recently selected a potential best-in-class novel orally available cGAS inhibitor small molecule Development Candidate known as IMSB301. We expect to advance this molecule to Phase 1 randomized placebo-controlled, double-blinded clinical trial in healthy volunteer subjects in 2024. We are evaluating IMSB301 and other novel small molecule cGAS inhibitors for potential applications in a broad range of inflammatory and autoimmune diseases, including CNS diseases with profound unmet medical need.
IMSA101
IMSA101, a novel small molecule analogue of cGAMP, is our lead product candidate and is the first STING agonist to be advanced to randomized Phase 2 clinical studies. Designed with a unique defense-sensing mechanism that increases anti-tumor immunity to ultimately stimulate cytokines to turn cold tumors to hot, IMSA101 holds first-in-class potential for treating solid tumors.
In preclinical studies, IMSA101 showed promise in both monotherapy and immunotherapy combination settings, demonstrating robust tumor growth inhibition as a single agent and with an anti-PD-L1 in multiple mouse models. In these early studies, IMSA101 stimulated production of IFNs and cytokines and generated long-term memory immunity to tumors.
We are currently conducting randomized Phase 2 studies evaluating IMSA101 in combination with hyper fractionated radiation and immune checkpoint inhibitors in patients with oligoprogressive (OPD) and oligometastatic (OMD) solid tumor malignancies. ImmuneSensor is the first company to advance a novel STING agonist to a randomized Phase 2 clinical study.
Drug Conjugate Platform
We are developing second-generation STING agonist drug candidates that are designed for targeted delivery of a STING agonist using a drug conjugate approach. We have developed a drug conjugate technology platform that conjugates, in a site-specific manner, highly potent STING agonists to antibodies or other molecules directed at proven cancer targets. The resulting immune stimulating drug candidate enables the delivery of STING agonists to the targeted area.
IMSA201 is our lead candidate from our drug conjugate technology platform and targets the clinically validated epidermal growth factor receptor (EGFR). In preclinical models, IMSA201 exhibited strong efficacy, activated myeloid cells and promoted intrinsic anti-tumor immunity.